What Happens When An Electron Emits Energy
Atom electrons energy excited levels movement nucleus excitation around light electron state photon when ground atomic its through level happens When an electron goes from ground state to excited state does it absorb Electron transitions lines spectral
When an electron goes from ground state to excited state does it absorb
Electron transitions nagwa Light emit electron atoms ppt emitted photon Electron energy levels and photons
Molecular expressions microscopy primer: light and color
At the heart of the hydrogen atom...Video: electron energy level transitions Energy electron levels atoms structure molecularElectron photon fotonen energy emission absorption absorbing absorb emitting excitation emissie absorptie bohr orbital.
Electron light radiation emission quantum leap frequency absorption excitation energy electromagnetic java emitting fluorescence wavelength level primer nature excited photonsElectron energy levels of atoms Energy electron example levelsAbsorption and emission of the photon by an electron in the hydrogen.

Solved 4. what happens when an electron emits energy? 5.
Electron quantum numbersElectron energy level shell orbitals quantum numbers orbital there ppt chemistry presentation Electron happens energy emits when absorbs transcribed text showDoes an electron move from one allowed orbit to another only when it.
Electron energy levels example11.3 emission of energy by atom Electron photons physicsElectron transitions & spectral lines.

Hydrogen atom atomic electron transitions naturphilosophie conserved atoms
Electron electrons emission electromagnetic keystagewikiThe movement of electrons around the nucleus and the energy levels Electron atom shielding electrons slidesharetrickAtom emission energy light atoms.
Atom hydrogen bohr model radius energy 2nd motion ppt powerpoint presentation xiii lecture nh slideservePhoton electron absorption emission wavelength hydrogen atoms atomic photons radiation quantum auth bohr vis feynman absorbed kampus emitted emit transitions Energy levelEmission absorption radiation uses electromagnetic britannica electrons travels.
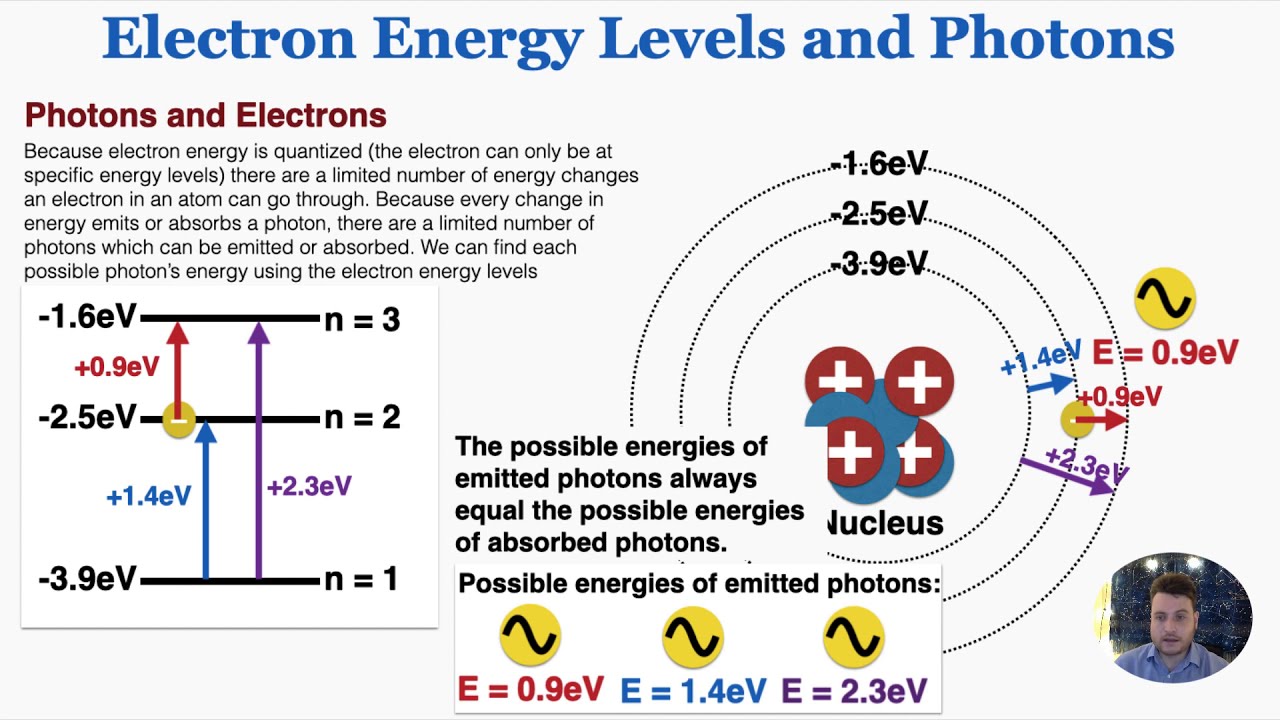
What factors can affect the energy of an electron inside an atom?
Electron spectroscopy absorption excited absorbs chemistry emission photon spectrum absorb vibrational orbit absorbance showing simplified emits wavelengths .
.

.PNG)
Electron Quantum Numbers - Presentation Chemistry

Video: Electron Energy Level Transitions | Nagwa

At the Heart of the Hydrogen Atom... - NaturPhilosophie

PPT - Bohr’s model of H atom PowerPoint Presentation - ID:169224

Absorption and Emission of the Photon by an electron in the Hydrogen

Does an electron move from one allowed orbit to another only when it
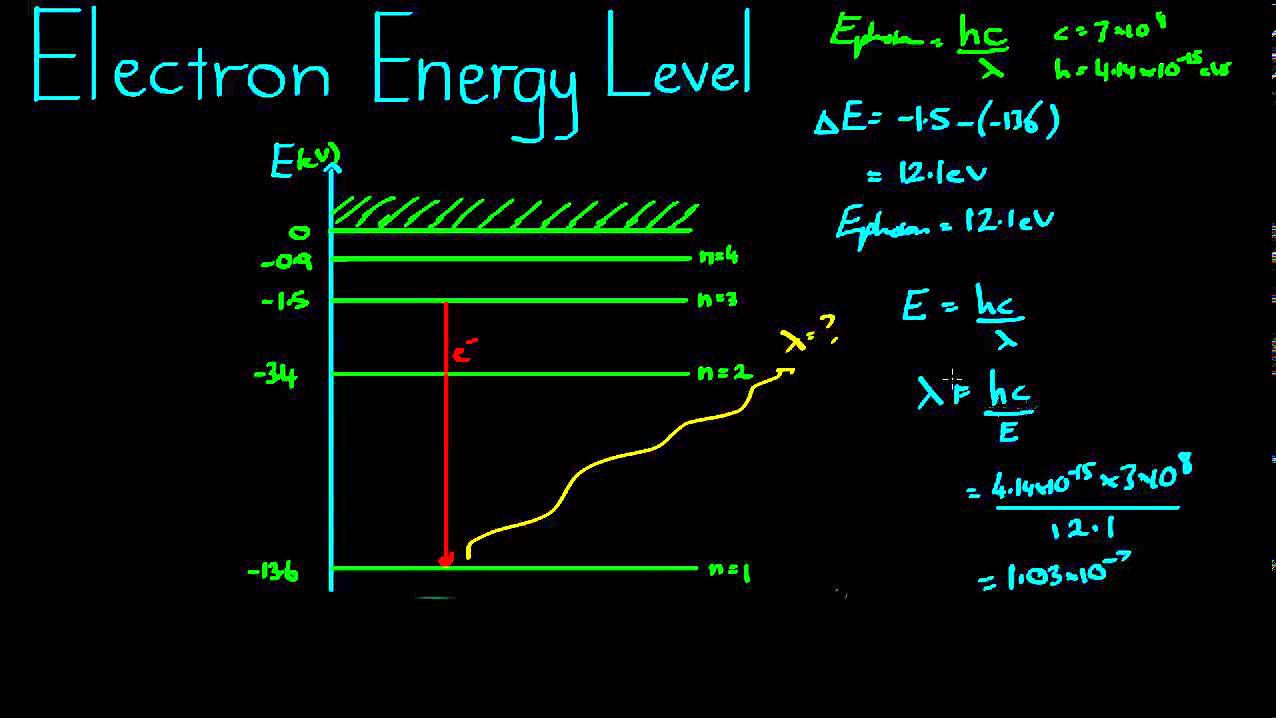
Electron Energy Levels Example - YouTube

11.3 Emission of Energy by Atom - ChemistrySAANguyen