How Many Orbitals In 3d Sublevel
Orbitals shapes atomic quantum chemistry atoms chem wave electrons theory shape electron numbers atom model chart orbital cartesian diagram edurev Why is 4s orbital filled before 3d orbital? Orbitals sublevel shapes sublevels 2s 3s axis identical made
s,p,d,f Orbitals - Chemistry | Socratic
Orbitals atomic atom sublevels structure electron shapes sub energy chemistry modern elements sublevel shape electrons levels level model configurations theory Electrons electron energy sublevels number level sublevel table orbital configuration chlorine each many periodic chart chem hold chemistry configurations does Orbitals each sublevels many following diagram orbital represents above box
Orbitals 3d representation chemistry chem libretexts electronic figure
Electron orbitalsOrbital electron shells occurred 2.2: electron configurationsWhy is the electron configuration of chromium "[ar]3d"^5"4s"^1" and not.
6.6: 3d representation of orbitalsElectron configurations orbitals sublevel each has line orbital chemistry box within Orbitals chemistry 3d look 3dz electron arrangement spd 3dx electronsElectron configuration orbital chart diagram sublevel atom circle representation fill each.

How do you represent electron orbitals through drawings?
Orbital orbitals quantum 5f atomic number magnetic electron 4f shapes chemistry types difference between seven atom shape different lobes chemieThe electron What is the electron configuration of chlorine?Orbital orbitals subshell symmetry 6h socratic materikimia kuantum bilangan introduced.
Sublevel 4s aufbau socraticShapes of orbitals and sublevels Orbitals orbital quantum sublevels atomic explained spdf parsing bonding hybridization answerShapes of orbitals and their types.

Electron configuration chart
Filling electrons order shell number maximum chemistry electron each which orbital 4s 3d filled why orbitals sublevels fill transition atomElectron configuration Orbitals chemistry electron atoms subshell order table atomic configurations periodic number structure quantum subshells electronic electrons energies which full configurationSpdf orbitals : parsing spdf orbital hybridization and simple bonding.
Atomic orbitalsSublevels (s, p, d, f) S,p,d,f orbitalsOrbitals 3d physics atomic nutshell.

Solved: chapter 7 problem 43qp solution
1.5-sublevels orbitals and electronsElectron configuration energy rule level orbital 5p 4d 4s fill 5s 6s diagrams presentation Orbitals , wave functions and quantum numbers class 11 notesThe sublevel.
Orbitals sublevels electronsOrbitals atom electron electrons quantum atoms subshell subshells represent chemistry orbital 2p shells majors socratic molecules isotopes ions energy L=4 subshell??.


Shapes of Orbitals and their Types | Chemistry Skills
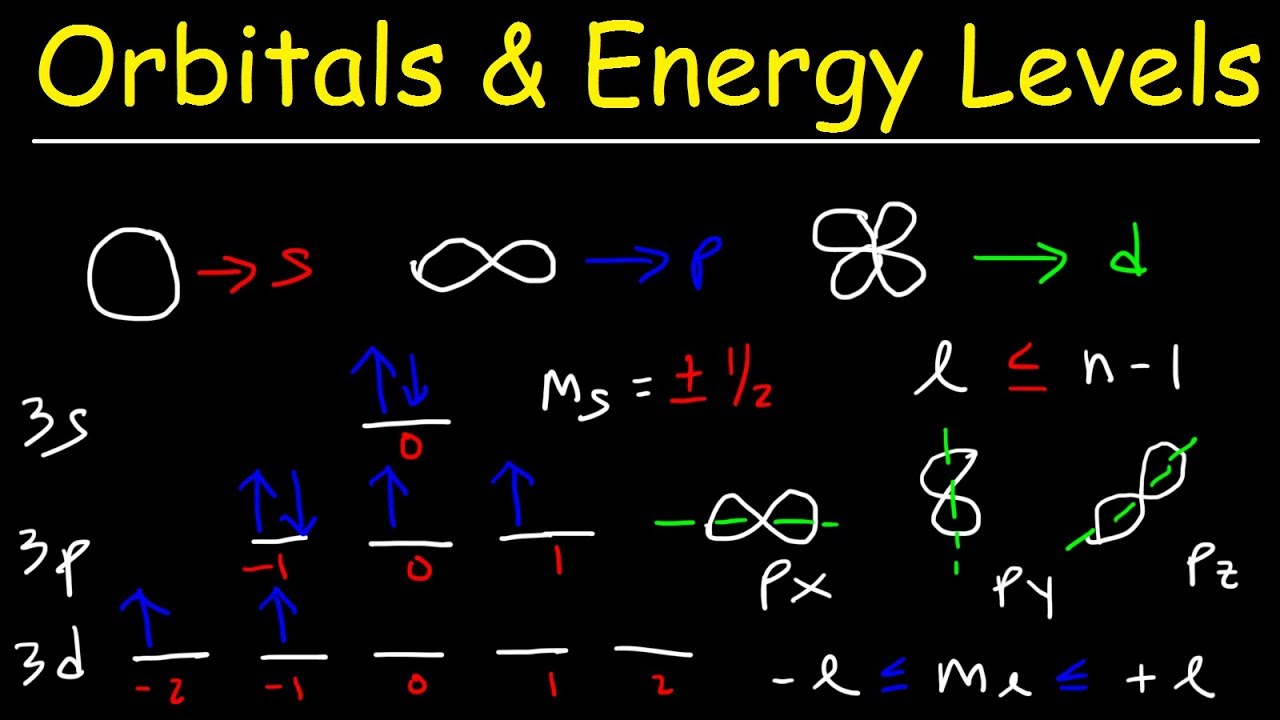
Spdf Orbitals : Parsing Spdf Orbital Hybridization And Simple Bonding

Sublevels (S, P, D, F)
/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)
Electron Configuration Chart

Why is 4s orbital filled before 3d orbital? - eNotes.com

Electron Orbitals | Definition, Subshells & Shapes - Lesson | Study.com

1.5-sublevels orbitals and electrons - YouTube
![Why is the electron configuration of chromium "[Ar]3d"^5"4s"^1" and not](https://i2.wp.com/useruploads.socratic.org/GzpxqflTSTm9KwkzEqYw_2-aufbau-principle.jpg)
Why is the electron configuration of chromium "[Ar]3d"^5"4s"^1" and not
