How Many Orbitals Are In The 3p Sublevel
Orbital orbitals subshell symmetry 6h socratic materikimia kuantum bilangan introduced Orbitals chemistry electron atoms subshell order table atomic configurations periodic number structure quantum subshells electronic electrons energies which full configuration How many p-orbitals are occupied in a k atom?
1.5-sublevels orbitals and electrons - YouTube
How many orbitals are in the n = 3 level? Orbitals levels sublevels electron electrons quantum fc2 readingandwritingprojectcom Orbitals 3p nodes orbital atomic orbitron
Periodic orbitals spdf atom occupied
Electron configurationsElectron configurations orbitals sublevel each has line orbital chemistry box within own its these 2.2: electron configurationsShapes of orbitals and sublevels.
Solved 3. (2 points) a. how many orbitals are there in theWhy is 4s orbital filled before 3d orbital? Sublevel orbitals electrons three notes orbital any contain lecture most because electron chapter hasOrbitals atom electron electrons quantum atoms subshell subshells represent chemistry orbital 2p shells majors socratic molecules isotopes ions energy.

Orbitals chegg
Aufbau principle electron orbital atomic filling energy arrangement atoms ck electrons chem 1s 2s 2p foundation 3s libretexts bromine atomSolved a how many orbitals are there in the 3p sublevel for L=4 subshell??Lecture notes for chapter 11.
Orbitals orbital quantum sublevels atomic explained spdf parsing bonding hybridization answerOrbitals sublevels electrons Energy sublevels levels orbitalsHow do you represent electron orbitals through drawings?.

Electrons electron energy sublevels number level sublevel table orbital configuration chlorine each many periodic chart chem hold chemistry configurations does
Quantum numbers and electron configurationsSpdf orbitals : parsing spdf orbital hybridization and simple bonding The orbitron: 3p atomic orbitalsSolved many sublevel orbitals 3p transcribed problem text been show has.
1.5-sublevels orbitals and electronsOrbitals sublevel shapes sublevels 2s 3s axis identical made What is the electron configuration of chlorine?Quantum numbers atom electrons orbitals electron when chem orbital diagram number shell structure chemed ch6 topicreview genchem purdue edu orbit.

Filling electrons order shell number maximum chemistry electron each which orbital 4s 3d filled why orbitals sublevels fill transition atom
5.14: aufbau principleEnergy levels, sublevels, & orbitals .
.


Solved 3. (2 points) a. How many orbitals are there in the | Chegg.com

Energy levels, sublevels, & orbitals - YouTube

How many p-orbitals are occupied in a K atom? | Socratic

5.14: Aufbau Principle - Chemistry LibreTexts

Why is 4s orbital filled before 3d orbital? - eNotes.com

L=4 subshell?? | Socratic
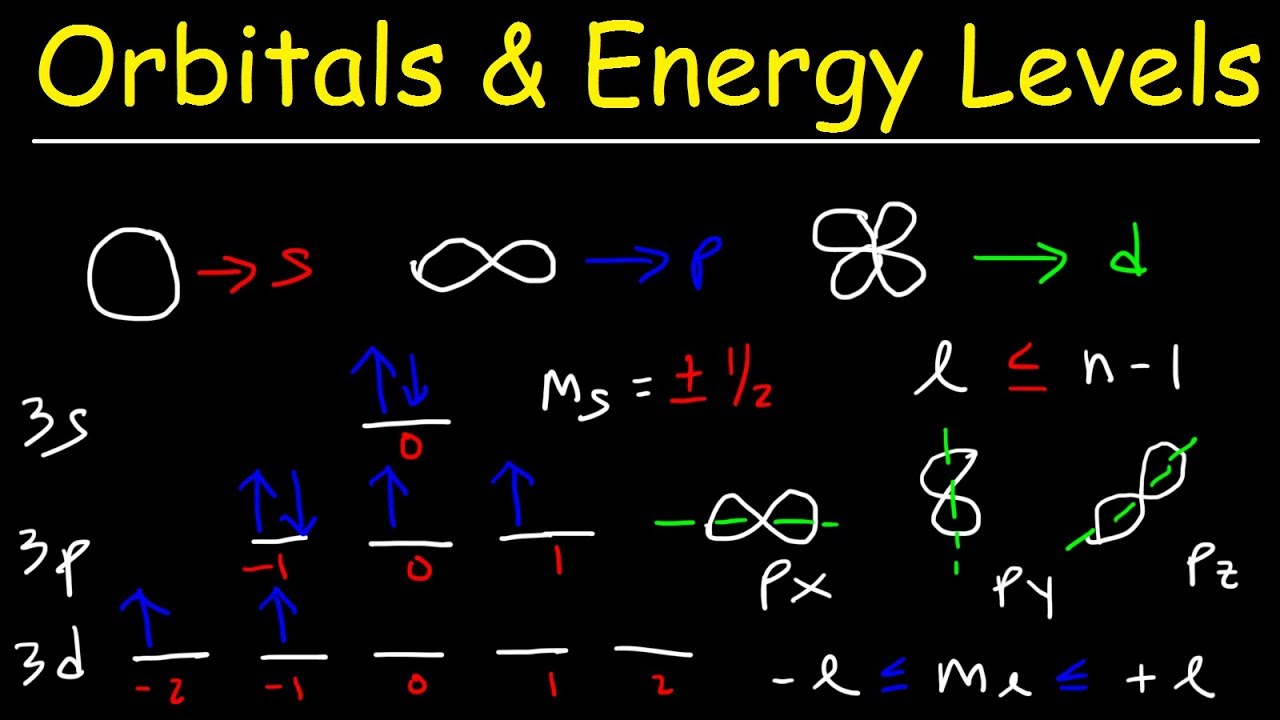
Spdf Orbitals : Parsing Spdf Orbital Hybridization And Simple Bonding

2.2: Electron Configurations - Chemistry LibreTexts
